Hi friends, in this article, I am going to explain the electroplating process steps and hope you will find it very informative and interesting.
The electroplating is an art of depositing a superior or more noble metal on a base metal by means of electrolysis.
For example, metals like iron are coated with deposits of nickel or chromium by electroplating to protect it from corrosion. Picture frames and machinery parts are often chromium-plated to protect them from corrosion and at the same time to give them a good appearance.
Electroplating Process Steps
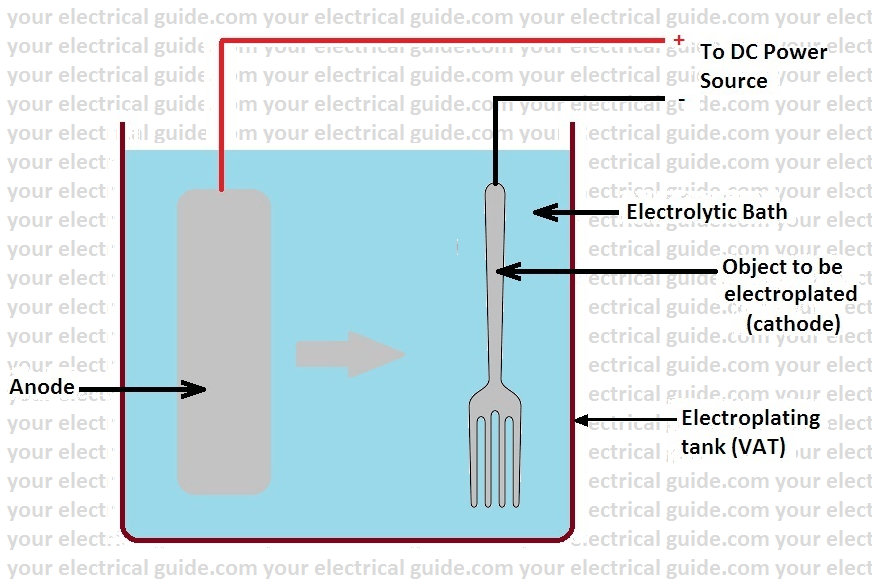
In an electroplating process, the object to be electroplated is made the cathode (i.e. connected to the negative terminal of DC source) in the solution of a salt of the coating metal.
The articles which are to be electroplated are suspended into the plating solution. The anode is also generally of the same metal. This arrangement is connected to a DC power source.
When DC supply is applied to the two electrodes, current starts flowing through the electrolyte. The metal ions begin to move towards the article and get deposited on it. The voltage requirement is small usually of the order of 1 to 16 volts only.
Cleaning Process
The surface on which process of electroplating is to be carried out must be polished and free of grease, scale, rust and dirt.
In case, the object to be electroplated is not cleaned, polished and degreased, the deposit formed may not well adhere to the base metal and is likely to peel off.
Oils and grease can be removed with the help of soaps, hot alkali solutions or solvents like CTC and gasoline. Rust, scale and oxides can be removed with the help of various acid, alkali and salt solutions mechanical abrasion and electrolytic cleaning in hot alkali solutions.
The articles are rinsed or water dipped between every process of cleaning such as physical cleaning, chemical cleaning, acid dips in order to prevent the carrying over of one processing solution over to the other.
To meet this requirement in every plating shop, special rinsing tanks with running water is extensively used. So ample supply of water with drainage facilities are to be provided in a plating shop. From the above discussion it is clear that cleaning is one of the most important electroplating process step.
Governing Factors of Electroplating Process
The factors, on which quality of deposit formed in electroplating depends, are given below:
- Nature of Electrolyte: The formation of a smooth deposit largely depends upon the nature of electrolyte employed. The electrolytes from which complex ions can be obtained, such as cyanides, provides a smooth deposit.
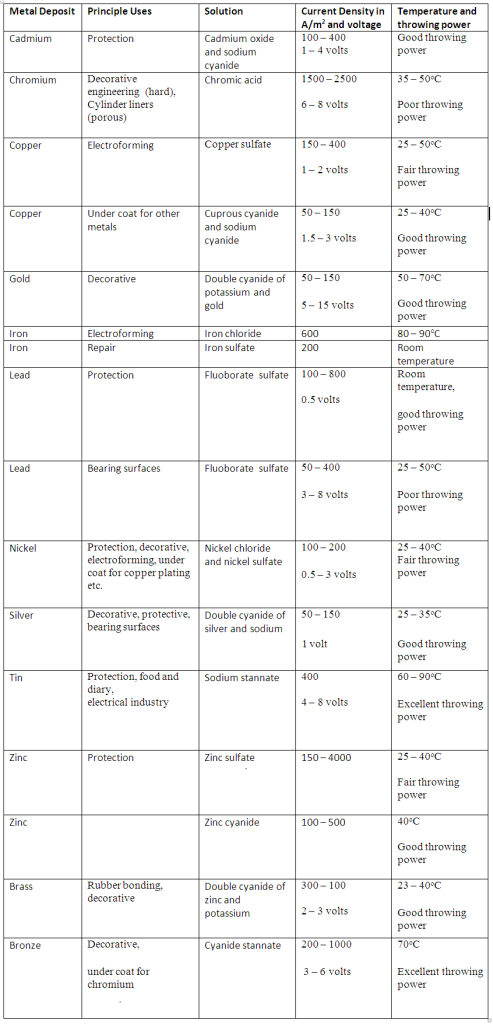
- Current Density: The appropriate current densities for different electroplating processes are shown in the table. At these current densities, the deposit of metal will be uniform and fine-grained. At the other current densities, the deposits will be coarse and crystalline in nature.
- Temperature: A low temperature of the solution favors formation of small crystals of metals and a high temperature, large crystals. In some cases, this is very marked a difference of only 15oC resulting in a 50% decrease in strength of metal deposited. On the other hand, a high temperature may give beneficial results due to increased solubility of salts and increased conductivity.
- Conductivity: The use of a solution of good conductivity is important from standpoint of view of the economy in power consumption and also because it reduces the tendency to form trees and rough deposits.
- Addition Agents: These are the substances which take little or no direct part in chemical reactions but influence the nature of deposits. When these are added to electrolytes promote the formation of small crystals and smooth deposits and at the same time permit the use of higher current densities. They also assist in producing a bright finish. The substances in use are glue, gelatin, albumin, glucose, dextral, phenol, glycerin, gum and many others.
- Throwing Power: This is the ability of electrolyte to produce a uniform deposit on an object of irregular shape.
The distance between the various portions of the cathode (object) and anode will be different due to the irregular shape of the object to be electroplated.
Due to unequal distance, the resistance of the current path through the electrolyte for various portions of the object will be different but the potential difference between the anode and the object will be the same and the result will be that the current density will be more on the portion nearer to the anode. It will cause an uneven deposit of metal.
The throwing power can be improved in two ways – firstly by increasing the distance between anode and cathode secondly by reducing the voltage drop at the cathode surface.
- Polarization: The rate of deposition of metal increases with the increase in electroplating current density up to a certain limit after which the increase in current density does not cause an increase in the rate of deposition.
Use of current densities beyond this limit causes electrolysis of water and hydrogen will deposit on the cathode. The hydrogen evolved blackens the base metal which diminishes the rate of metal deposition. This phenomenon is known as polarization. Blackening effect of the base metal can be reduced by agitating the electrolyte. Therefore, while performing the “electroplating process steps”, appropriate current densities should be used.
With reverse current electroplating, in which at regular intervals plating current is reversed for a second or so, the polarization effect becomes negligible even with the very high overall speed of plating. Unsound and inferior metal is depleted during the reverse current period and flat level surfaces are produced. The metal surface is brightened causing the elimination of buffing or polishing operation.
- Voltage: The voltage required to pass the current through any electrolyte in the process of electroplating is equal to the sum of voltage drop in the resistance of the electrolyte and the voltage drop at the electrodes (cathode and anode).
From the resistivity of the electrolyte and the x-sectional area and the length of the electric current path, the resistance of electrolyte can be determined.
To economize on power, the electrolyte resistance should be reduced to the minimum and to achieve it special conducting agents are added in many cases. The voltage per cell varies from a fraction of volt to about 6V.
There is always some potential difference between the cathode and electrolyte and between the electrolyte and anode. This potential difference is a measure of the tendency of the metal to go into the solution and is known as electrode potential.
The electrode potential depends upon the exact conditions (i.e. temperature and concentration) and also upon the nature of metal and the electrolyte. Under ideal conditions, the value of electrode potential for most of the substances lies between 0.5 to 1 volt.
Electrolytic Bath
The electrolyte used in electrolytic bath depends upon the nature of the metal to be deposited. The electrolytic deposits provided by the process of electroplating are crystalline in nature.
The crystals must be very fine in order to get firm, coherent and uniform deposits. For this purpose, suitable electrolytes should be used in electrolytic bath and current density used should have an appropriate value. The temperature should also be maintained at a proper level.
By experiments, a certain optimum value of current density and temperature has been worked out for each electrolyte. The optimum values of current densities and temperatures for different electroplating processes are shown in the table.
For copper plating, two types of electrolytic baths are used.
- In an acid type bath, the solution is made of 150 – 200 gm of copper sulfate and 25 – 35 gm of sulfuric acid per 1000 cc of solution. Deposit obtained is thick and rough requiring polishing.
- In cyanide bath, solution is made of 25 gm of copper cyanide, 28 gm of sodium cyanide, 6 gm of sodium carbonate and 6 gm of sodium bi-sulfate per 1000 cc of solution. It provides thin and smooth deposits.
Copper anodes are used in both of bathes.
For silver plating, the solution is made of 24 gm of silver cyanide, 24 gm of potassium carbonate and 36 gm of potassium cyanide per 1000 cc of solution.
For gold plating, the solution is made of 18 gm of potassium gold cyanide, 12 gm of potassium cyanide, 6 gm of potassium sulfate and 12 gm of caustic potash per 1000 cc of solution. Anode employed is of stainless steel.
For chromium plating, the solution is made of 180 – 300 gm of chromic acid and 2 – 3 gm of sulfuric acid per 1000 cc of solution. Higher current densities are used for hard chromium plating than for decorative chromium plating. Anodes are of antimonial lead. Vats used for chromium plating are of steel with lead lining. Chromic acid is added in solution when required.
For nickel plating, the solution is made of 180 – 240 gm of nickel sulfate, 36 gm of nickel chloride and 24 gm of boric acid per 1000 cc of solution. The anode is of pure nickel.
Nickel does not deposit well on iron and steel articles. Therefore, the article is first coated with a film of copper and then nickel is deposited on the copper plating.
Electroplating of Alloys
It is possible to deposit alloys also provided the electrode potentials of the constituent metals are not much different.
For deposition of alloys, the anode is made of an alloy to be deposited and electrolyte consists of a mixture of electrolytes which would have been employed for separate deposition of the metals consumed.
Electroplating of brass is an example of it. For the brass electroplating, a solution of double cyanide of zinc and potassium and of copper and potassium is used as the electrolyte. The current density of 25 – 40 A/m2 is used.
Precautions in Electroplating Process
- The object to be electroplated must be cleaned thoroughly.
- It should not be handled with bare hands.
- All connections must be clean tight and free from corrosion.
- Keep the current constant during the process of electroplating.
- The plates must be rinsed in dilute H2SO4 after removing from the electrolyte, otherwise, the deposit will turn black.
Thanks for reading about electroplating process steps.
Utilization of Electrical Energy| All Posts
- Direct | Indirect Resistance Heating
- Induction Heating | Ajax Wyatt Furnace
- Coreless Induction Furnace
- Electric Arc Furnace Working Principle
- Dielectric Heating Principle
- Electrical Resistance Welding
- Describe the Electroplating Process
- Laser Beam Welding Process
- Electron Beam Welding Working Principle
- Domestic Refrigerator Working Principle
© www.yourelectricalguide.com/ electroplating process steps.